Sulfur Oxidation
(H2O2)
Examples:
Example 1
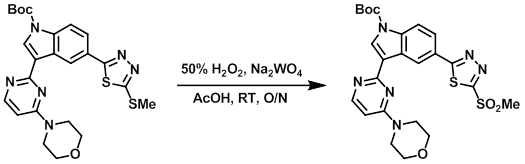
A mixture of the SM (1.7 g, 3.3 mmol), AcOH (15 mL), Na2WO4 (209 mg, 0.60 mmol), H2O2 (50%, 2.3 mL, 6.6 mmol) was stirred at RT overnight. The solvent was removed in vacuo and ice/H2O was added to the residue. The mixture was extracted with DCM. The combined organics were dried, concentrated, and purified by silica gel chromatography to provide the product. [1.65 g, 90%]
[Patent Reference: WO2012129338, page 91, ![]() (12.0 MB)]
(12.0 MB)]
Example 2
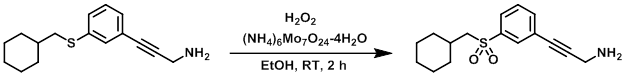
To a mixture of the SM (0.454 g, 1.59 mmol), (NH4)6Mo7O24-4H2O (0.585 g, 0.474 mmol), and absolute EtOH was added 30% H2O2 (1.6 mL, 15.7 mmol). The reaction mixture was stirred at RT for 2 h, after which time it was concentrated in vacuo. To the resulting residue was added H2O and the mixture was extracted with EtOAc (2x). The combined organics were washed with brine, dried (MgSO4), and concentrated to provide the product as a white solid. [0.471 g, 93%] [UK Pat App GB2463151A, page 120]
